High Throughput Kinetic Screening for Optimum Therapeutics
Determination of mAb binding kinetics (ka & kd) and affinity (KD) are essential to optimum therapeutic candidate selection. SPR is the gold standard in kinetic characterization of therapeutic mAbs, but other techniques are commonly used as a primary screening tool due to the low throughput, high sample requirements, and limited data analysis capability of current SPR instruments.
Our High Throughput SPR Platform Changes the Paradigm, Delivering High Throughput Kinetics mAb Screening:
- High throughput: Kinetically screen and rank up to 1,152 mAbs, 384 clones at one time in parallel
- Low sample usage: Only < 200µl of each interactant required
- Ease-of-use: Simple sample layouts prevent the need for complex plate maps and multiple aliquots of each reagent
- Rapid data analysis: Powerful software tools enable large data-sets to be rapidly fitted and visualized and automatically validated against several data quality parameters
- Detailed data mining: Interrogate mAb panels via interactive on-rate, off-rate, affinity and iso-affinity plots
- Data integration: Combine binning and kinetics screening data for un-paralleled mAb evaluation
Kinetic Analysis of 384 protein:protein Binding Interactions
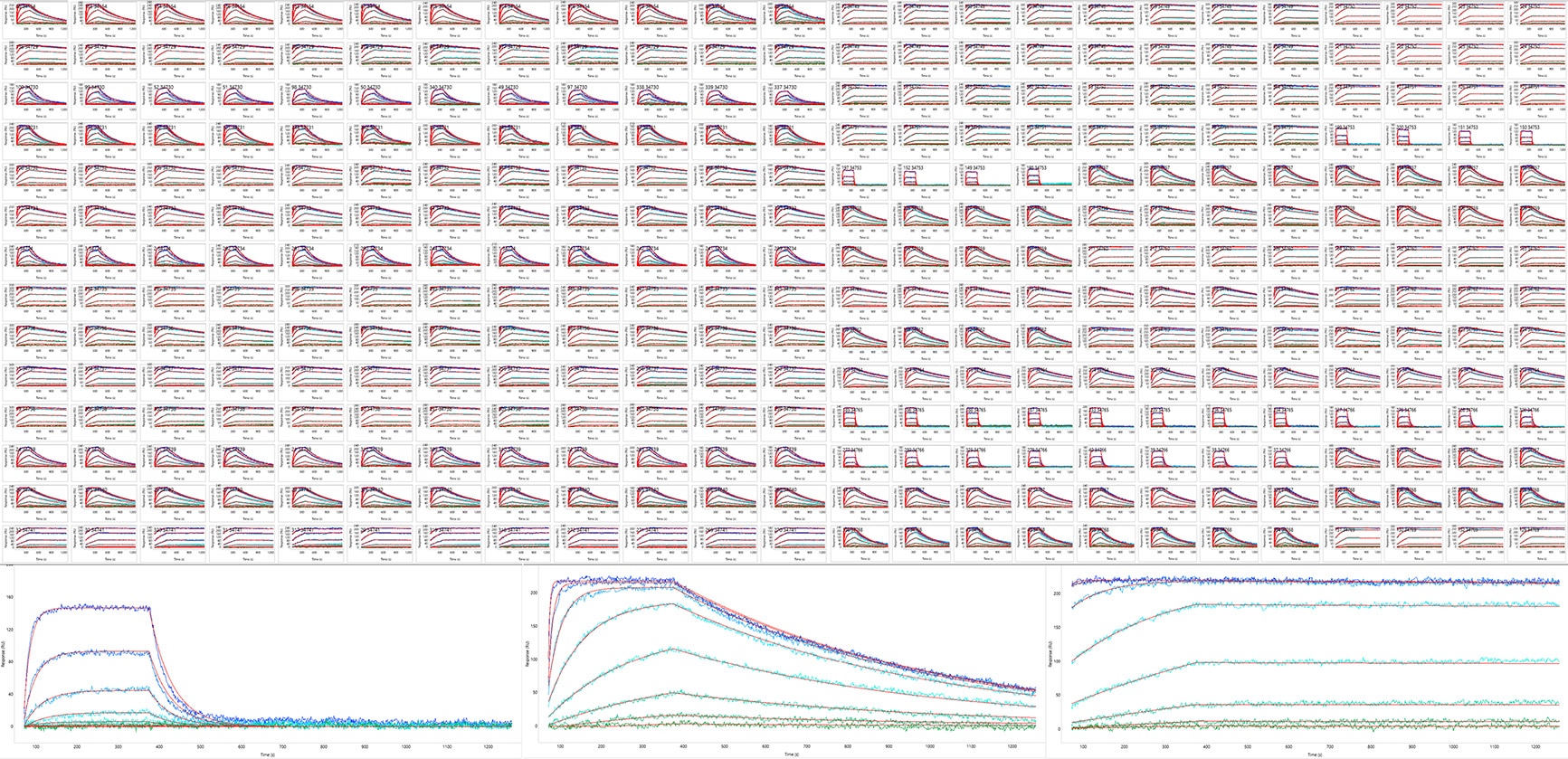 Simultaneous kinetic analysis of 384 antigen/antibody binding interactions using high throughput SPR. (Top) The results from a single capture kinetics assay where a monovalent target (analyte) was titrated over 384 antibodies, each captured onto individual spots of a 384-array. Each panel represents the global kinetic analysis on a single spot; all 384 spots were analyzed in parallel (some spots are excluded). (Bottom) Enlarged view of the data from 3 spots showing antibodies that bound their target with diverse kinetics (low, medium, and high affinities, from left to right).
Simultaneous kinetic analysis of 384 antigen/antibody binding interactions using high throughput SPR. (Top) The results from a single capture kinetics assay where a monovalent target (analyte) was titrated over 384 antibodies, each captured onto individual spots of a 384-array. Each panel represents the global kinetic analysis on a single spot; all 384 spots were analyzed in parallel (some spots are excluded). (Bottom) Enlarged view of the data from 3 spots showing antibodies that bound their target with diverse kinetics (low, medium, and high affinities, from left to right).
Determining Antibody Affinity
Both the on-rate (ka) and off-rate (kd) of antibodies are of equal importance in determining antibody affinity and potency. Off-rate ranking alone can fail to meaningfully differentiate large groups of candidates. Combining measures of association and dissociation kinetics in a single experiment yields more thoughtful data sets that can be rapidly meshed with other criteria for candidate ranking. High throughput kinetics facilitates robust selection of candidate antibodies with binding profiles better aligned to specific therapeutic objectives.
Strategies for High Throughput Binding and Kinetic Screening:
 Coupled kinetics on up to 384 clones per assay:
Coupled kinetics on up to 384 clones per assay:
- Couple antibodies directly to the sensor chip using proprietary flow printing technology and time-tested surface chemistries
- Probe with several concentrations of the antigen. Each antigen concentration is flowed over the entire array in parallel from a single 200 µL sample volume
- Minimal sample requirements: Typically, only 200 ng of each clone is required and roughly 100 pMoles of antigen (3 µg for a 30 kDa antigen)
- Easy set up: 1 plate with antibodies for immobilization, 1 well or tube for each concentration of antigen is all you need to prepare
 Capture Kinetics on up to 1,152 clones per unattended run:
Capture Kinetics on up to 1,152 clones per unattended run:
- Flow printing technology allows for extended capture contact time under high flow rates for highly efficient sample capture even from dilute crude primary samples such as cell culture supernatants and bacterial extracts. Captured samples are returned to the well and can be used for other analysis in most cases
- Capture up to 384 at a time, or 1,152 unique samples in a single automated run
- Prepare capture surfaces using a wide variety of approaches (Fc, Fab, polyhistidine, V5, etc.)
- Minimal antigen requirement: Roughly 100 pMoles (3 µg for a 30 kDa antigen) are needed for each 384 well set of antibodies
- The set up only requires the sample plates, a single tube for each antigen concentration, and a vial of regeneration solution for removing the captured mAbs from the array in between prints
You can get full kinetics (ka, kd, and KD) on 1,152 antibodies in one run, in a single day, from supernatants, with only 9 µg of antigen!
