High-Throughput SPR Accelerates Vaccine Discovery and Development
Carterra’s HT-SPR technology platform enables rapid, label-free characterization of hundreds of interactions in parallel, helping vaccine developers move from discovery to clinic faster than ever before.
Traditional SPR instruments are low throughput, analyzing one to a few interactions at a time. Carterra’s HT-SPR platforms change the game with array-based, high-throughput measurement and analysis, enabling researchers to screen, characterize, and rank hundreds of interactions in parallel, allowing understanding of the immunological response and epitope profile from a vaccine campaign in one step.
Highlights:
- Integration with mRNA, protein subunit, and viral vector vaccine workflows
- Screen hundreds of donor-derived samples
- Track immune responses in parallel
- Evaluate a large library of vaccine design variants for target epitope inclusion
- Characterize binding of hundreds of TCR/pMHC interactions
- Kinetics, affinity, epitope binning, and epitope mapping
- Real-time, label-free interaction analysis
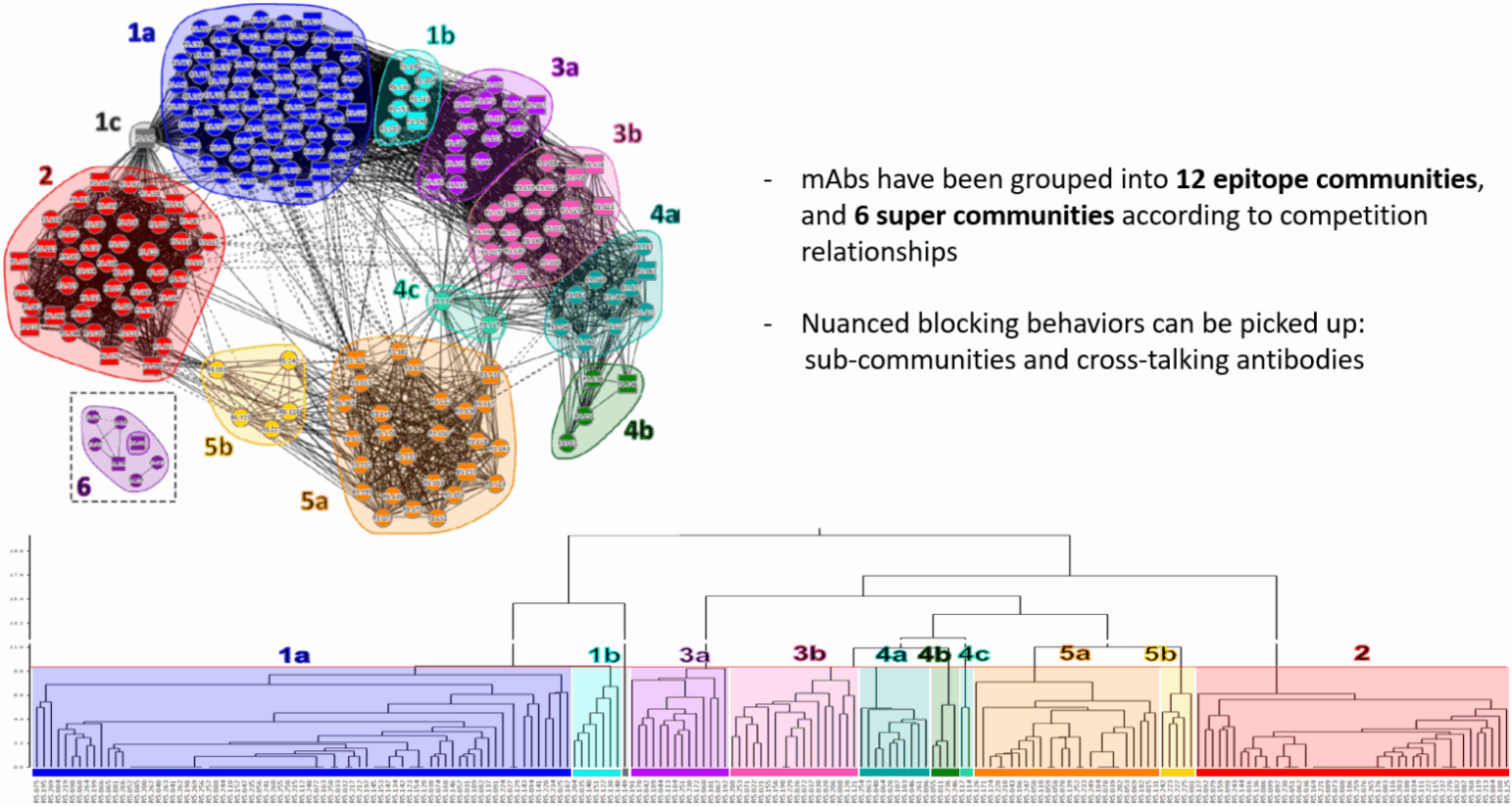
Figure 1. Combining epitope information with functionality, kinetics, and sequencing information reveals determinants of potency which will aid next-generation vaccine design — data from Malaria Vaccine Development, University of Oxford. Data generated on the Carterra LSA platform with analysis on Carterra’s Epitope Software.
Carterra’s Impact on Pandemic-Scale Vaccine Efforts
Carterra’s platforms were instrumental during the COVID-19 pandemic, allowing Eli Lilly and government agencies to characterize thousands of neutralizing antibodies in record time. Our platform technology is used by many organizations researching COVID-19, Herpes Simplex, and Malaria vaccine development programs, including the NIH, FDA, Regeneron, the University of Pennsylvania, and University of Oxford.
