
Technology
Carterra’s LSA instrument seamlessly integrates proprietary fluidics with SPR detection to deliver true array-based, high throughput label-free detection.
Microfluidics
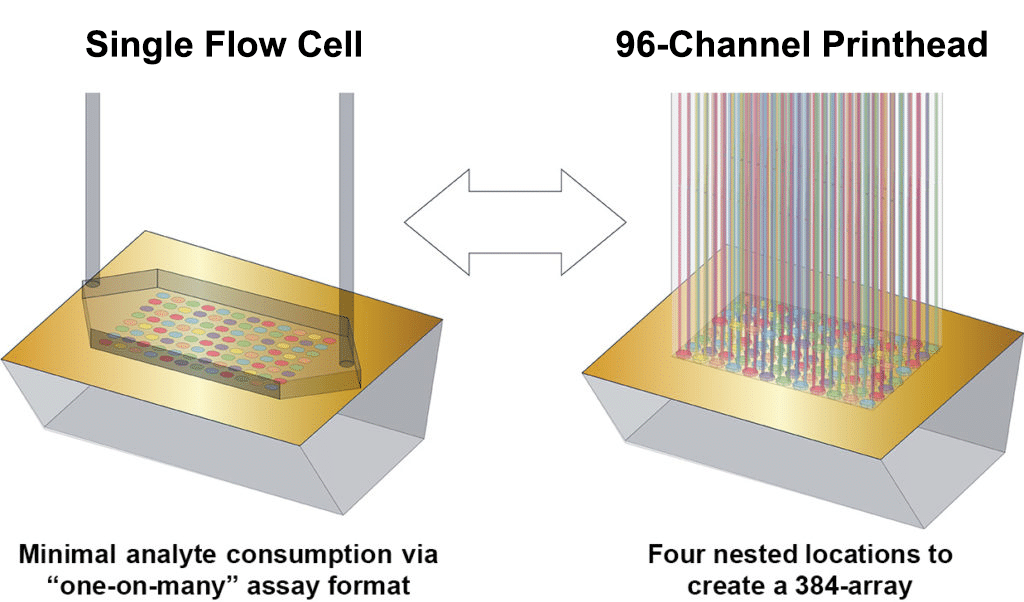
The LSA has two independent fluidic modules: a 96 Printhead based on Carterra’s patented flow printing technology for the building of arrays on chip surfaces; and a proprietary Single Flow Cell (SFC) that enables one sample or reagent to flow across the entire chip surface array. The LSA software automatically controls these two modules during an assay for complete hands-free operation.
Flow Printing
Carterra’s patented flow printing technology was invented in the lab of Prof. Bruce Gale at the University of Utah Department of Mechanical Engineering and uses a network of microchannels that uniquely enable flow-based array printing of proteins and antibodies on sensor surfaces. In contrast to spotter and printer technologies that use fixed volume droplets with uncertainty in binding coverage, flow printing uses micro-channels to flow and cycle ligand solutions over a defined surface area. This maintains sensitive proteins in a liquid environment throughout the immobilization step and produces highly defined active spots with coverage that approaches saturation.
Surface Plasmon Resonance (SPR) platforms typically use a unidirectional flow system that limits both association and dissociation times which impose restrictions on kinetic studies and increase sample usage. By turning the orientation of the flow cell through 90°, flow printing enables the same flow characteristics required for high quality kinetics studies, but with significant benefits in terms of fluidic performance and SPR data output, thereby expanding applications.
Key Benefits of Integrated Flow Printing/SPR Technology:
Higher throughput
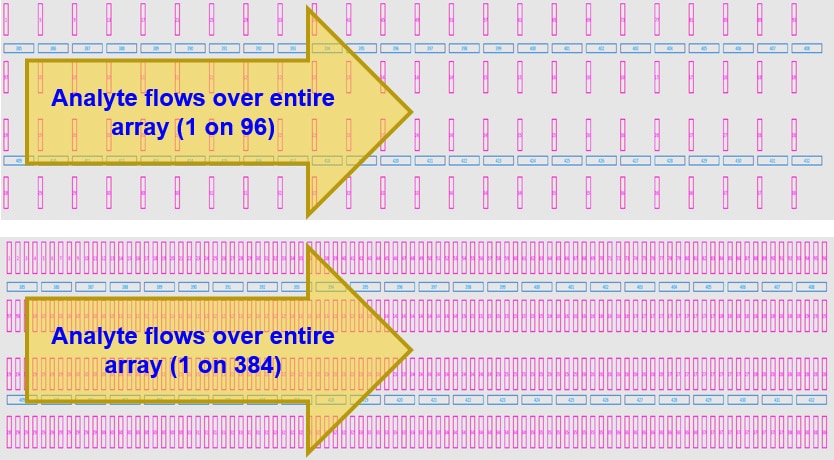
- Up to 384 immobilized spots within the SPR detection area, plus 48 local reference spots
Broad applicability
- Print antibodies, proteins, peptides, DNA and more
- Print ligands directly from crude mixture onto selective capture surfaces
Bidirectional flow
- Extended immobilization and dissociation times
- Reduced sample volume requirement
Increased sensitivity
- Print from dilute solutions, letting flow concentrate the material onto the surface
- Enables ligand concentrations 1,000x more dilute to be printed than other technologies
Fully enclosed and independent micro-channels
- Defined spot shape
- Prevents drying and denaturation during immobilization
- Deliver multiple reagents in series across each spot
- Highly reproducible spot-to-spot immobilization

The 96-Channel Printhead enables a 96 protein array to be immobilized in parallel from flow onto a sensor chip surface in a single step. Sample is taken from either a 96 well plate or one quadrant of a 384 well plate and cycled through the 96 Printhead which is docked onto the surface of an SPR chip. Each of the 96 printhead channels creates a fluidically isolated flow cell on the surface of the chip, enabling flow-based immobilization for 96 distinct samples simultaneously. This can be repeated up to 4 times to build a 384 array on the SPR surface, all with real-time SPR detection throughout the assay, from surface preparation through to kinetic interaction.

Single Flow Cell (SFC)
The LSA’s Single Flow Cell (SFC) utilizes a single needle and dual syringe pumps to deliver high-quality flow across the chip surface via a proprietary fluidics cartridge. The SFC cartridge automatically switches with the printhead to cover the entire chip surface as needed, for surface preparation, regeneration, and delivery of one sample to address all spots created on the surface by the 96 Printhead.
Surface Plasmon Resonance (SPR) Detection
The Carterra LSA utilizes Surface Plasmon Resonance to detect binding interactions in real-time for up to 384 samples in parallel. While traditional SPR biosensors have practical limitations in the number of unique measurable locations per surface area, imaging-based SPR can monitor hundreds of locations within a tightly constructed array, while still maintaining excellent sensitivity and data collection rates.
The LSA seamlessly integrated both single flow cell and 96-channel printhead switching.
The LSA utilizes a laser diode light source to illuminate the functionalized gold surface at the interface with the prism and reflected light is detected via a high-resolution CCD camera. At the boundary of the functionalized gold layer and the glass prism a certain fraction of incident light photons propagates as surface plasmons, forming an evanescent field which is sensitive to changes in refractive index (RI) at the functionalized surface. When the RI changes, such as when molecules bind, the angle of incident photon absorption shifts and this change in the minima of reflected light is used to quantify binding.
While the CCD camera in the LSA can monitor the entire SFC area, data collection is focused on locations by using flow printing technology (up to 384), as well as unprinted locations typically used as references (48), totaling 432 spots, over which a single analyte is then flowed.

Sensor Chips
LSA sensor chips consist of a functionalized gold wafer adhered to a dove-cut glass prism, forming an SPR coupler capable of eliciting the SPR phenomenon. Within the LSA, the sensor chip is docked in the path of the optics module allowing incident light to interact with this SPR coupler, while a portion exits as reflected light to the detector.
LSA Instrument: Integration of Flow Printing, SFC and SPR Technology
The LSA instrument integrates flow printing technology via the unique 96-channel printhead for array building; the SFC for full surface preparation and regeneration, as well as pulse-free sample delivery for high quality kinetics; with SPR array detection. From a user perspective, all this occurs via simple to use software “Apps” that make assay set up easy and fully automated for hands-free operation. The LSA automatically switches between the 96 Printhead and SFC as required by the specific protocol, while the SPR monitors surface interactions in real-time for the entirety of the assay.
Aviva Systems Biology Webinar – Immuno-oncology
Massively Parallel SPR Based Fragment Screening on Ligand Arrays
