Unlocking High-Throughput Biology and Drug Discovery – View Speaker Presentations
Industry and academic scientists presented new paradigms in discovery, applications and workflows including HT-SPR. Topics included:
- New methods in antibody discovery, DELs and PROTACs
- Innovations using HT-SPR for drug discovery
- Challenging assay formats, such as membrane targets and rapid binders
- Strategies to streamline characterization workflows
- Best practices in experimental design and analysis
The speakers included scientists from …

You can view the slides from some of the presentations below.
 Grant Murphy, PhD, Executive Director, Discovery Biologics, Merck
Grant Murphy, PhD, Executive Director, Discovery Biologics, Merck
Keynote Address—Leveraging computation and HTP experimentation to engineer biologics
Abstract: This talk will cover our efforts to combine computational modeling, HTP protein-protein interaction assays, and biophysical characterization to engineering improved biologics.
 Bill Harriman, PhD, SVP, Antibody Discovery, OmniAb
Bill Harriman, PhD, SVP, Antibody Discovery, OmniAb
Keynote Address—Use of high throughput antibody characterization to validate novel transgenic animal platforms
Abstract: Engineered animals designed to produce human sequence antibodies can be evaluated and compared via antibody repertoire analysis at the nucleic acid level, however this approach does not capture antibody specificity profiles and “functional diversity” that may be present at the paratope level. We have generated over 20 different genetic configurations during the past decade, and have routinely evaluated the corresponding immune repertoires through the functional analysis of panels of antigen-specific mAbs using high throughput epitope binning and kinetics analysis. This type of characterization is also critical downstream, in workflows focused on hit expansion and antibody optimization that leverage deep phenotypic screening, NGS datasets, and structural modeling methods.
 Sharon L. Schendel, PhD, Program Manager, La Jolla Institute for Immunology
Sharon L. Schendel, PhD, Program Manager, La Jolla Institute for Immunology
Painting a Landscape: Defining nuanced epitope communities targeted by antibodies against SARS-CoV-2 spike protein
Abstract: The Coronavirus Immunotherapeutics Consortium (CoVIC) is an international effort to conduct side-by-side, apples-to-apples comparison of leading therapeutic antibody candidates against the SARs-CoV-2 spike protein that were contributed by a range of large and small companies as well as academic groups on multiple continents. The CoVIC panel includes over 400 antibodies that originated from a variety of sources, including COVID-19 survivors and in silico design. All antibodies were sent to seven different partner reference labs for analyses of different antibody features, including in vivo protection in a mouse model of infection, affinity for spike protein, high resolution epitope binning, ability to block ACE-2 binding, neutralization of pseudovirus and authentic virus infection in cell culture, and Fc-mediated immune effector function. All data generated by the reference labs are accessible in the public database CoVIC-DB (https://covicdb.lji.org/). High-throughput, high resolution binning of CoVIC antibodies carried out using the Carterra LSA platform was particularly critical for defining the broad and nuanced landscape of antibody epitopes on the SARS-CoV-2 spike protein. This binning helped define epitopes that are associated with durable potency against multiple SARS-CoV-2 variants of concern, including Omicron, and also highlighted the competition profile of the CoVIC antibody panel. Results of CoVIC studies can be used to guide isolation of effective antibody therapeutics, selection of durable therapeutic cocktails, immunogen design.
 Christian Stegmann, PhD, Senior Vice President, Drug Creation, Absci
Christian Stegmann, PhD, Senior Vice President, Drug Creation, Absci
Amir Shanehsazzadeh, Senior AI Scientist, Absci
Therapeutic Antibody Design Using Generative AI
Abstract: Artificial intelligence (AI) has the potential to greatly increase the speed, quality and controllability of drug creation. We showcase such potential on two antibody drug creation tasks: de novo design and lead optimization. For the first task, we utilize generative AI models for zero shot design of antibody CDRs. In particular, we screen over 1 million antibody variants designed for binding to human epidermal growth factor receptor 2 (HER2) using our high-throughput wet lab capabilities. Our models successfully design all CDRs in the heavy chain of the antibody and compute likelihoods that are calibrated with binding. We achieve binding rates that are several multiples higher than HCDR3s and HCDR123s randomly sampled from the Observed Antibody Space (OAS). We further characterize 421 AI-designed binders using surface plasmon resonance (SPR), finding three that bind tighter than the therapeutic antibody trastuzumab. The binders are highly diverse, have low sequence identity to known antibodies, and adopt variable structural conformations. For the second task, we show that our high-throughput screening assay can generate quantitative binding affinity scores for hundreds of thousands of antibody variants. After validating these scores with SPR, we use this data to train large language models that accurately predict binding affinities for unseen antibody variants. These models can be used to co-optimize multiple antibody properties, as we demonstrate by designing variants of trastuzumab with up to seven mutations and with over ten-fold increase in binding affinity. Combined, these approaches promise to accelerate and improve antibody drug creation, and may increase the success rates in developing novel antibody and related drug candidates.
 Kendra Avery, PhD, Associate Director, Xencor
Kendra Avery, PhD, Associate Director, Xencor
Discovery and characterization of Fvs for Anti-CD28 bi- and tri-specific antibodies to treat solid tumors
Abstract: T cells in the tumor microenvironment (TME) require both signal one and signal two for complete activation. Providing tumor targeted, anti-CD28, signal 2 may enhance the complete activation of T cells in the TME to provide a robust anti-tumor effect. In this presentation, I will describe some of the discovery and characterization efforts of the tumor associated antigen (TAA) arms of XmAb808, an Anti-B7-H3 x Anti-CD28 2+1 antibody, currently in phase 1 clinical trials for a wide variety of solid tumor types, and Anti-PD-L1 x Anti-PD-L2 x Anti-CD28, a tri-specific antibody in pre-clinical development.
 Zachary V. Fagiani, Associate Scientist, Dragonfly Therapeutics
Zachary V. Fagiani, Associate Scientist, Dragonfly Therapeutics
High-throughput SPR assay for FcγR binding to drug candidates on the new Carterra LSAXT
Abstract: Fc gamma receptor (FcγR) binding is a critical component of ADCC, ADCP, and CDC and an important feature to measure in Fc-containing drugs. The purpose of our study was to determine if testing IgG drugs against a full panel of biotinylated FcγRs can be completed on Carterra’s LSAXTinstrument with higher throughput than a traditional 8-channel biosensor while maintaining equivalent data quality. Our results show that the LSAXT demonstrates highly comparable data relative to the traditional 8-channel biosensor while using 7.5 times less analyte, 6 fewer hours of analyst time, ~$3,000 less in consumables, and producing 8 times as many replicates.
 Zara Frizell, PhD, Associate Scientist, Sanofi
Zara Frizell, PhD, Associate Scientist, Sanofi
How Carterra LSA enables high-throughput binding assessment of NANOBODY® molecules
Abstract: The variable domain of heavy chain-only antibodies from camelids, referred to as NANOBODY molecule, offers numerous advantages for the development of biological drugs. Sanofi Ghent has pioneered the development of this new modality. Following the classical drug development process, one of the most important parameters we are looking at in early screenings is the binding strength of the NANOBODY molecule to its target. This talk will highlight practical aspects and considerations to set up NANOBODY-specific binding assays on the Carterra LSA that have become broadly applicable during lead identification and characterization steps in the Discovery department of Sanofi Ghent.
 Matthew Greving, PhD, VP, Machine Learning and Platform Technologies, iBio
Matthew Greving, PhD, VP, Machine Learning and Platform Technologies, iBio
High-throughput SPR to screen and train machine learning designed antibody libraries
Abstract: Antibody discovery efficiency and quality can be enhanced with machine learning. A lack of high-quality activity data at a scale sufficient to test and train models is a major limitation in the integration of machine learning and antibody discovery. We will present the use of high throughput Carterra LSA binding screens to test activity and accuracy of machine learning designed antibody libraries. In addition, we will present the use of high throughput Carterra LSA binding screens to train a machine learning model that predicts high-affinity antibodies for a target.
 Carterra Application Scientists
Carterra Application Scientists
Discovery and characterization of multi-specific antibodies: New capabilities and workflow
Abstract: Selection of bi- and tri-specific binders often require the screening of large combinatorial sample sets derived from panels of single domain binders. The Carterra LSA platform makes analyzing the binding properties of these molecules straightforward and requires minimal amounts of mAb sample and antigen. The affinity of the binders to the targets can be measured in several assay formats and used to verify the independence and activity of each binding site for hundreds of clones. Along with the binding kinetics and specificity measurements, the LSA enables large scale epitope binning to ensure diverse sets of clones are being carried forward to functional evaluation.
 Carterra Application Scientists
Carterra Application Scientists
Leveraging the new Carterra LSAXT for challenging HT-SPR applications
Abstract: The LSAXT expands the capabilities of HT-SPR to meet the demands of challenging assay formats. By building on the established benefits of HT-SPR including more data in less time with less sample, LSAXT has the versatility to address a range of experimental workflows. This talk will highlight how LSAXT is a robust platform for characterization of drug candidates including PROTAC®s and kinase inhibitors. The benefits of enhanced sensitivity and overall data quality in LSAXT take HT-SPR to a whole new level.
“The location was great, plenty of refreshments and I liked how there was a good mix of networking and talks.” –Cambridge UK 2023 Attendee
“It was diverse, engaging, and very informative.”–Zurich 2023 Attendee
“This symposium was incredibly informative and the networking was incredible.”–San Francisco 2023 Attendee
“Good location and content was great.”–Boston 2023 Attendee
To learn more about Carterra and our offerings contact us using the following link: Contact us

Kathryn Hastie, PhD, Instructor, at Ollmann Saphire Lab, La Jolla Institute for Immunology, gave the keynote address at the San Diego, CA Symposium.

Gemma Ottolangui, Investigator from GSK, shared her presentation on “High-throughput screening and data handling in the antibody discovery process” at the Cambridge, UK Symposium.
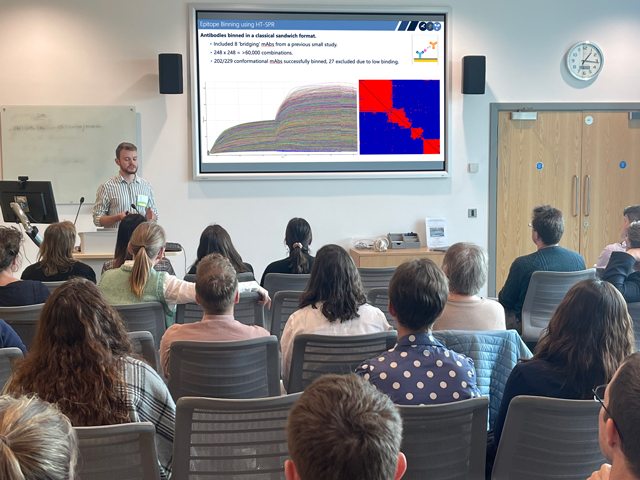
Jordan Barrett, Senior Research Assistant from the University of Oxford, also shared his presentation on “High-throughput isolation and characterisation of monoclonal antibodies against PfRH5 – the leading blood-stage malaria vaccine candidate” at the Cambridge Symposium.

Presenters from various companies shared applications and workflows at the San Francisco, CA symposium.

Christian Stegmann, PhD, Senior Vice President, Drug Creation at Absci, shared his presentation on
“Therapeutic Antibody Design Using Generative AI” in Zürich, Switzerland.
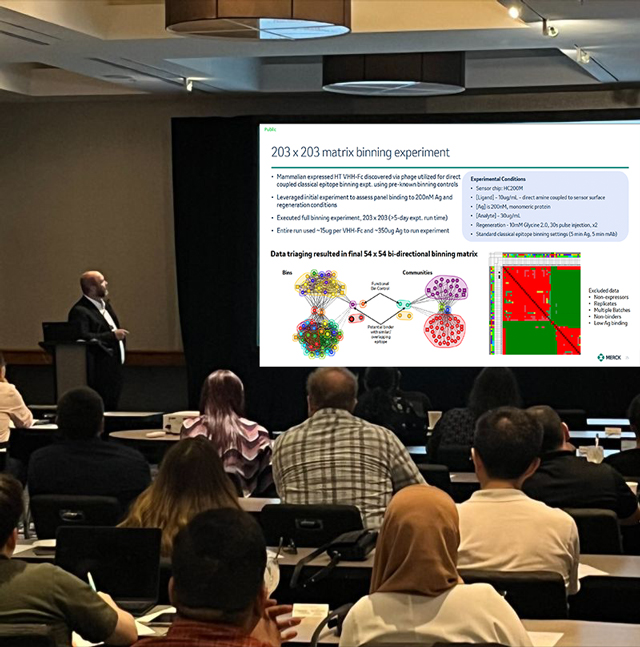
Grant Murphy, PhD, from Merck, gave the keynote address at the Boston, MA symposium.

Attendees enjoyed networking with peers during the multiple breaks and lunch.

Symposia speakers and Carterra team members enjoyed dinner the night before the Zürich Symposium.
